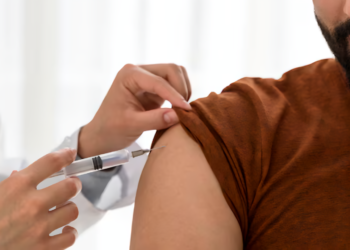
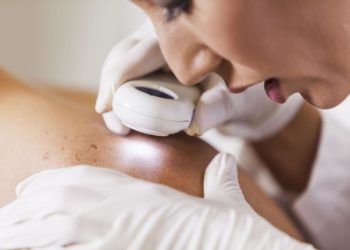
ReadyGo Diagnostics Ltd. has introduced an advanced single-tube assay for detecting the Monkeypox (Mpox) virus, marking a significant development in rapid diagnostic solutions. This new assay is designed to meet the urgent demand for quick and precise Mpox testing.
The assay offers results in under 20 minutes and is compatible with ReadyGo’s SnapCollect sample collection system, which excels at processing saliva samples. It can be used with ReadyGo’s cost-effective Geo molecular platform or as an independent kit suitable for traditional laboratory molecular systems. The assay employs Meridian Bioscience Inc.’s Air-Dryable Direct RNA/DNA LAMP Saliva (MDX137) reagents to deliver high accuracy and reliability, which are crucial for managing patient care and controlling outbreaks.
Set to undergo extensive validation to meet global standards, the assay is expected to become available this month. It will be provided to healthcare professionals and partners, particularly in areas where affordable point-of-care diagnostics are necessary. This innovative tool aims to enhance early detection and management of Mpox, supporting global health efforts.
ReadyGo Diagnostic’s CEO, Dr. Ben Cobb, stated: “Our innovative approach harnesses the latest advancements in diagnostic technology to meet the urgent needs of healthcare providers worldwide.”
He continued: “We have been working with Meridian’s innovative molecular reagents for some time and our combined expertise will bring a much-needed powerful Mpox detection tool to the market.”




![7 Best POS Software in the UK [2026 Edition]](https://todaynews.co.uk/wp-content/uploads/2026/02/7-Best-POS-Software-in-the-UK-2026-Edition-360x180.png)