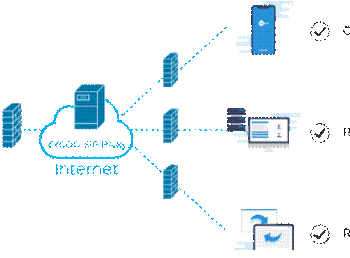
Introduction: Why Certification Matters
Certification is more than a regulatory formality. It is a confirmation that a medical device has met essential safety, performance, and quality requirements before entering the market. Whether you are a manufacturer, healthcare provider, or procurement officer, verifying that a medical device is certified is a critical step in ensuring compliance and patient safety.
Uncertified or falsely labeled devices pose real risks. They may not meet necessary technical or clinical standards, and in many markets, their use is outright illegal. That’s why knowing how to check a medical device’s certification status isn’t optional.
What Does “Certified” Mean for a Medical Device?
Certification confirms that a medical device complies with the regulations of the region where it is marketed. In the European Union, this typically means CE marking under the EU Medical Device Regulation (MDR). In the United States, it refers to FDA clearance or approval under the applicable classification.
This process often involves conformity assessment by an independent body. It includes risk management, clinical evaluation, documentation review, and evidence of compliance with a quality management system such as ISO 13485. A certified device has been reviewed to ensure it meets both safety and performance criteria for its intended use.
Steps to Check If a Device Is Certified
There are several ways to verify the certification status of a medical device. Start by inspecting the product itself. Certified devices should have a visible regulatory mark, such as a CE symbol (often followed by a four-digit number identifying the notified body) or an FDA listing number. These marks are usually found on the packaging or the device label.
Next, request the Declaration of Conformity from the manufacturer. This is a formal document stating that the device meets the relevant regulatory standards and has undergone the required conformity assessment. For FDA-regulated products, you can also search the FDA Registration and Listing Database to verify clearance or approval.
In the EU, the EUDAMED database will eventually allow public access to certification information, but until then, third-party auditing bodies or manufacturers themselves may be the best sources. A reliable supplier will provide up-to-date technical documentation upon request.
Understanding how a certified device is developed can also help in the verification process.
Key Global Certification Marks to Recognize
Different regions have different certification systems. The most common include:
- CE Marking (European Union): Indicates conformity with EU MDR or IVDR. It is mandatory for most medical devices sold in the EU.
- FDA Clearance/Approval (United States): Devices are either “cleared” (510(k)) or “approved” (PMA) depending on risk class.
- Health Canada License Number (Canada): Class II, III, and IV devices must have a medical device license.
- TGA Approval (Australia): Devices must be included in the Australian Register of Therapeutic Goods (ARTG).
- Japan PMDA Approval: Devices in Japan must be approved by the Pharmaceuticals and Medical Devices Agency (PMDA).
Each of these marks indicates that the device has undergone a formal review process. Absence or misuse of these symbols is a red flag.
How Internal Audits and QMS Support Certification Status
Certification is not a one-time event. It must be supported by an ongoing quality management system. ISO 13485 is the global standard for medical device QMS and is often required for certification in regulated markets.
Regular internal audits are part of maintaining this system. They ensure that all aspects of production, documentation, and post-market activities remain compliant with regulatory expectations. These audits also detect problems before they affect the safety or reliability of the device.
For companies involved in medical device development or supply, training internal auditors can strengthen compliance efforts. The ISO 13485 Internal Auditor Training Course provides essential tools to conduct effective audits aligned with certification requirements.
Common Pitfalls: When a Device Isn’t Actually Certified
Not all devices that appear certified truly are. Some may display fake CE marks or expired certificates. Others may be imported through unauthorized distributors who bypass local conformity procedures. In some cases, accessories or software components are used with certified devices but are not certified themselves.
Another issue is scope. A certificate may only apply to a specific model or configuration. If a device has been modified or used outside of its intended purpose, it may no longer be covered under its original certification.
Always confirm the authenticity of certification documents and verify the notified body or regulatory authority involved. If something feels unclear, contact the manufacturer directly or check public databases when available.
Conclusion:
Knowing how to check if a medical device is certified is essential in today’s regulatory landscape. It ensures legal compliance, protects patients, and builds trust in the healthcare supply chain. Certification marks, supporting documents, and public databases all provide transparency, but diligence is required to interpret them correctly.
A certified medical device is not just labeled for sale, it has been reviewed, tested, and audited to meet international standards. Whether you are sourcing devices, developing products, or managing quality systems, don’t assume compliance. Verify it.


![7 Best POS Software in the UK [2026 Edition]](https://todaynews.co.uk/wp-content/uploads/2026/02/7-Best-POS-Software-in-the-UK-2026-Edition-360x180.png)